Business Intelligence
Clario’s unified reporting system for consistent trial oversight
We know the challenges of running a successful clinical trial across multiple sites, partners, and various devices require centralized oversight. At the center of our clinical trial management and document, system are performance reports for guidance in making insightful operational decisions.
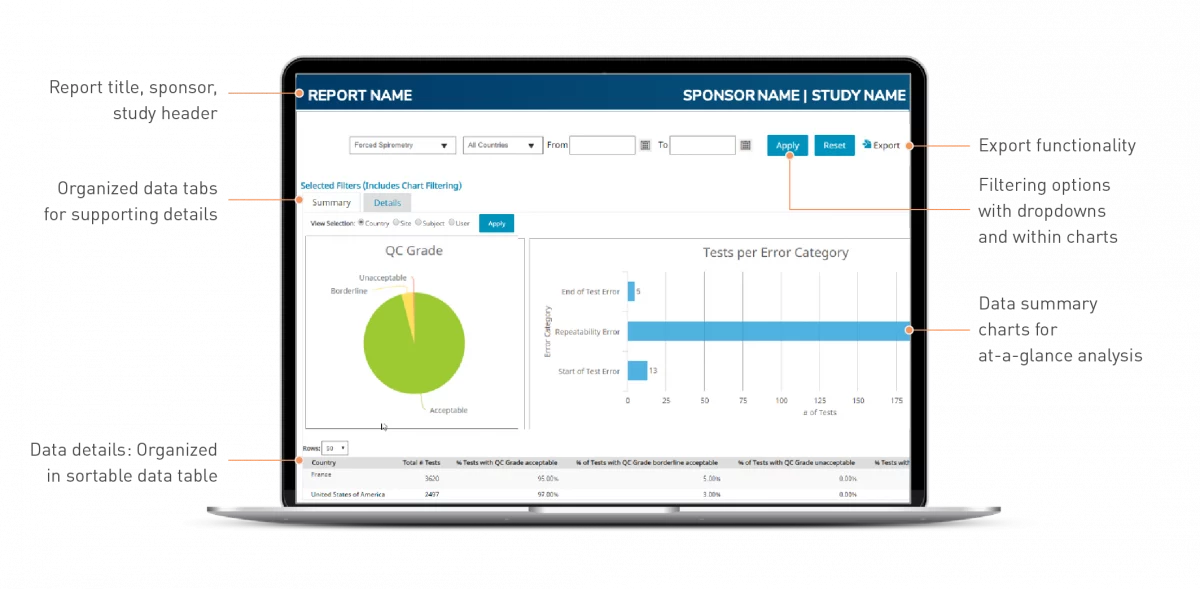
Data collection reports
Quickly access and interact with endpoint evidence
- eCOA and imaging compliance
- Cardiac safety test quality
- Respiratory measurements
Study progress reports
Monitor the progress and health of a study
- Visit tracking and completion
- Subject enrollment and inclusion details
Engagement reports
Monitor engagement and communication
- Customer care tickets
- Evidence correction queries
- Study contact information
- User login and report activity

Gain insight from:
- Single point access to summarized evidence and detailed information
- Tailored reports to manage projects and maintain quality and compliance
- Identifying issues that could impact trial performance
Data insights
Manage data quality and compliance with automated insight and detection.
Next best action: focused, targeted problem solution
Our fundamental approach to clinical trial risk management starts with presenting answers, not questions. By applying advanced analytics to our Endpoint and Safety data, we can provide visibility on where problems might exist so that you can take action. Data Insights is a clinical trial data management system that removes the time spent looking for problems and repurposes that time to solving them.
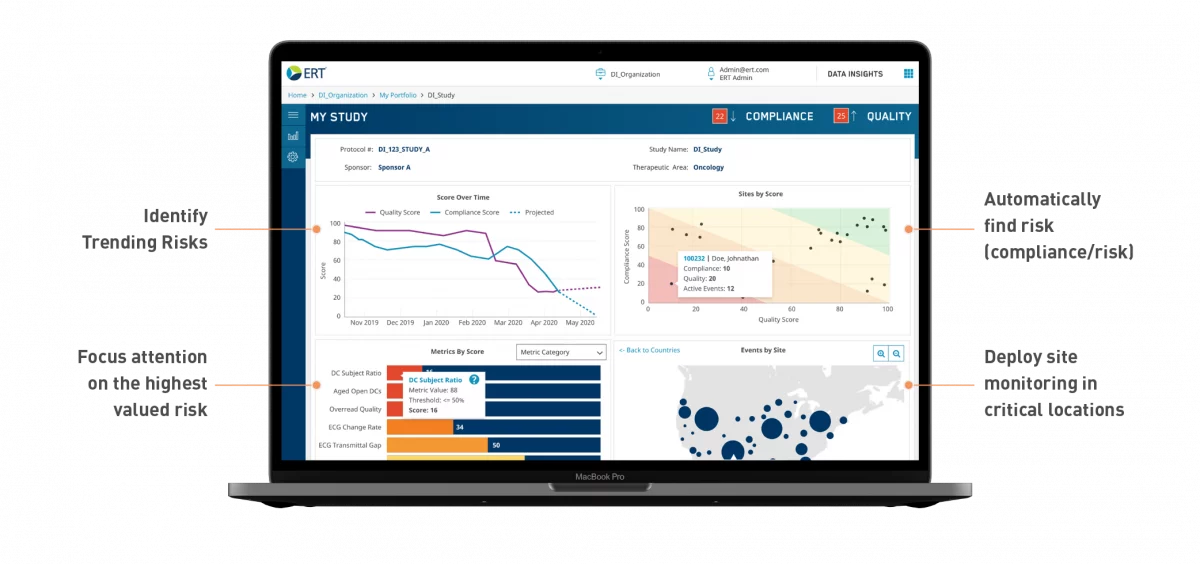

Take confident and decisive action
Act before risks become issues to trial outcomes and data quality.
Gain trust in your data quality and compliance through enhanced visibility across the study portfolio with our clinical trial data management system.
Prioritize workflow for quicker issue resolution and cost savings.
Reduce effort through automation and intuitive visualization that time to solving them.
Data exchange
Inbound and outbound data integration with Sponsor, CRO and 3rd Party systems to analyze, and monitor for data quality and trial compliance in support of surveillance and oversight.
As our industry strengthens its focus on Data Analytics, one of the most significant barriers for our customers in reaching their goals is access to data. You cannot unlock the value of data without full transparency and accessibility. Clario has made a significant investment in our clinical trial data analysis and management infrastructure to meet our customers’ unique data services needs in answer to this challenge.
Automated file transfers solutions allow companies to:
Monitor and track all file transfers from a central platform
Securely and reliably exchange of critical data
Maintain visibility overall file transfer activity
Meet and maintain security and compliance requirements
Data transfer solutions offered
sFTP
Clario automatically transfers data configured to your specifications
Real-time data transfer
Provide Clario data to 3rd party systems in a near real-time data stream.
API
Receive Clario data on-demand from Clario systems over scalable RESTful web service.