Reduce queries and costs
Approximately 20% of queries will need to be reopened if you submit DICOM images to a clinical trial without a pre-submission image screening system. In some hospitals, the query rate jumps to 80%! These queries cost time and money — and take dollars from valuable research funds. Reduce your costs with pre-submission image screening AI technology, including custom, trial-specific acceptance criteria.
Protect patient privacy
Improper de-identification of DICOM images can result in visible patient health information (PHI), which risks patient privacy. Our SMART Submit clinical research image processing software uses over 150 rules to detect and rectify common issues in DICOM files, including the presence of PHI.
Reduce the risk of image quality issues
SMART Submit’s proprietary automated de-identification system involves three levels of verification and is 48-fold more proficient at removing PHI than other DICOM transport solutions. The research site loads the original DICOM into the software application, enters the case trial identifiers, and SMART Submit handles the rest.
Increase efficiency
Our clinical research imaging experts perform quality control (QC) for anomalous cases or studies generated by new imaging equipment. As part of our image QC check, our team can save you time by querying the site directly to resolve any image issues or screen files for your specified acceptance criteria.
Customer support you can count on
Our multilingual customer support team is available to assist you anywhere in the world with image uploads, transfers and viewing 24/7/365. Our image QC technicians, data managers and DevOps teams are dedicated to image management to help keep your project moving.
How does it work?
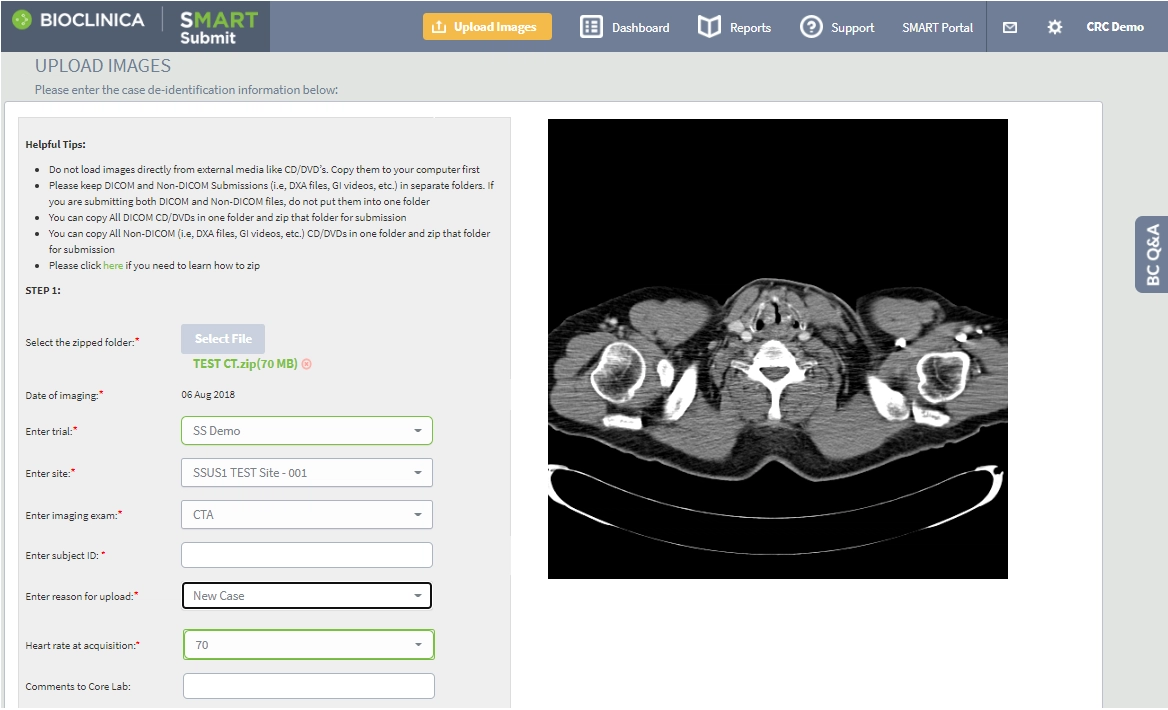
- Easy upload: Upload an image from anywhere in the world in just a few clicks with our clinical research imaging platform.
- Nothing to install: Upload images using our secure, 100% browser-based Global Cloud Network — no additional hardware or software required.
- Accessible: Give sponsors or authorized users access to the images online as soon as they are de-identified. Send images automatically to one or more recipients, download them on demand or store them in our online Image Repository.
Easily store and share
21 CFR Part 11 and EU GDPR-compliant images anywhere in the world from any device for use in future clinical trial projects or audits.
Talk to a specialist
Our team of experts is available to address questions you may have. Submit your contact information and we’ll be in touch shortly.