OVERVIEW
Cough monitoring
Cough monitoring has been moving away from subjective patient self-reporting in favor of more objective and quantitative measures. As regulators continue to look for more granular cough and lung sound metrics, we continue to partner with device manufacturers to bring sponsors the most advanced respiratory trial solutions to meet these needs.
For which indications is cough typically assessed?
Cough and lung sounds are evaluated for a number of respiratory diseases:
- Asthma
- Chronic Obstructive Pulmonary Disease (COPD)
- Chronic bronchitis
- Cardiopulmonary diseases
- Allergies
- Gastroesophageal Reflux Disease (GERD)
- Sleep disorders and nocturnal wheezing
- Postnasal drip
SOLUTION FOR
Objective cough and lung sound monitoring
Clario supports accurate, in-home monitoring of cough and lung sounds through RESP® Biosensor, a lightweight, wearable device able to capture 24 hours of continuous cough recordings. Used in combination with the Clario iSpiro® home spirometry solution, the RESP Biosensor enables sponsors to gain objective cough metrics alongside spirometry and eCOA data.
RESP® Biosensor System
Allows for the following endpoints:
- Cough count, severity and spasms
- Lung sounds: wheezing, crackles, rhonchi, snore and others
- On-board accelerometer allows for concurrent measurements*:
- Respiratory rate and heart rate
- Activity levels
- Body positioning
Benefits of the Clario and Strados Labs Solution:
- iSpiro, RESP and eCOA applications all on the same handheld device
- Continuous recording of up to 25 hours on a single charge
- Small, comfortable, hands-free device with no wires
- Clinically validated, FDA 510(k) Cleared, CE Marked Device
- Validated overread process supported by machine learning algorithms*
- HIPAA-compliant, encrypted data storage and transfer

Patient-friendly wireless device
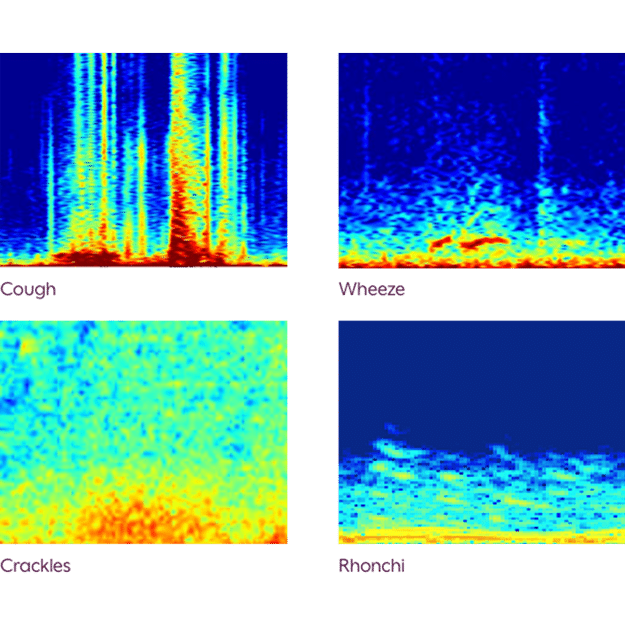
Lung sound events are displayed visually as spectrograms to facilitate the overread process*
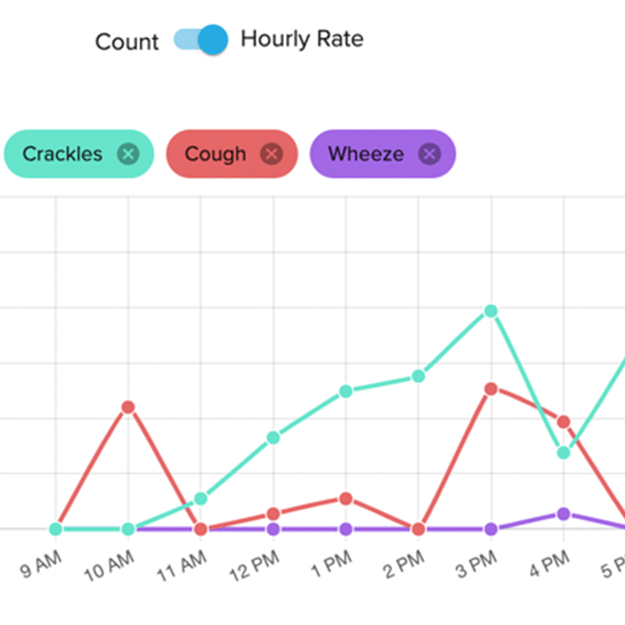
Granular data over a 24-hour period with concurrent measurement of various respiratory endpoints
*Algorithms, respiratory rate and chest excursions not yet FDA cleared
RESP Biosensor metrics at-a-glance
1st
510(k)-cleared wearable device for lung sounds
24h
continuous recording
19M
breaths captured
600k
validated coughs
OVERVIEW VIDEO
Learn more about the benefits of Strados Labs’ RESP Biosensor System in clinical trials
Related solutions
Talk to a specialist
Our team of experts is available to address questions you may have about our Respiratory solutions. Submit your contact information and we’ll be in touch shortly.



