OVERVIEW
AI in clinical trials
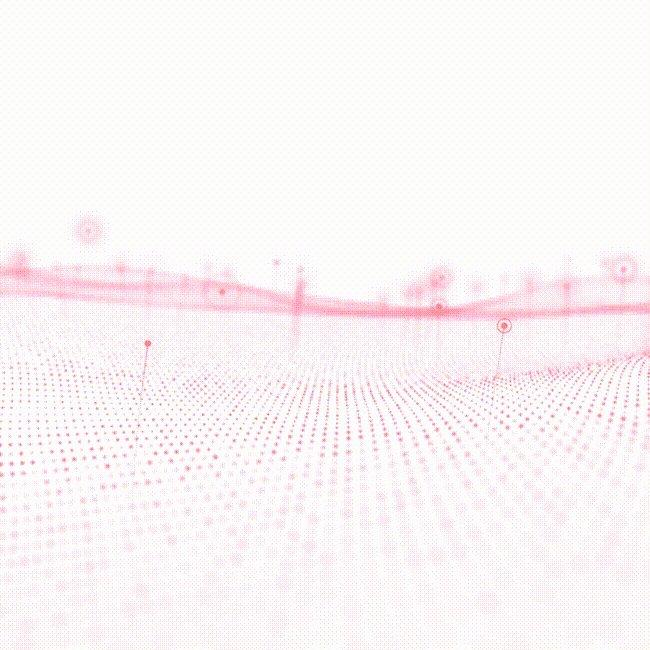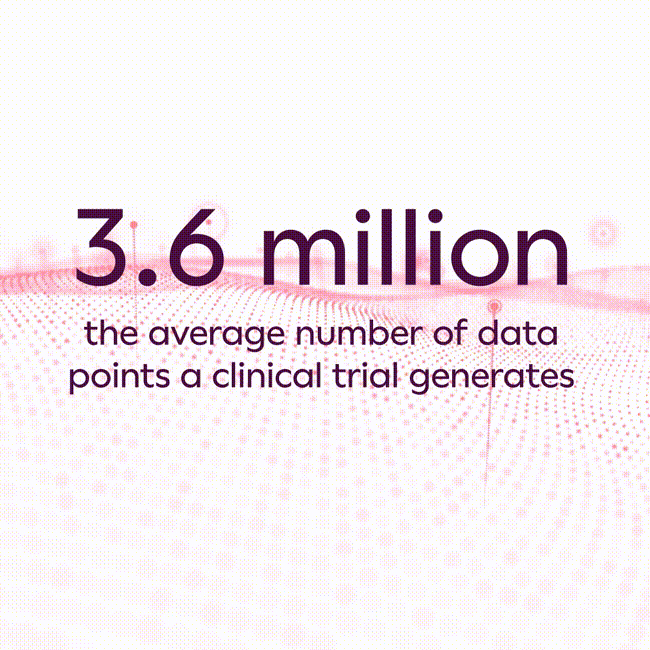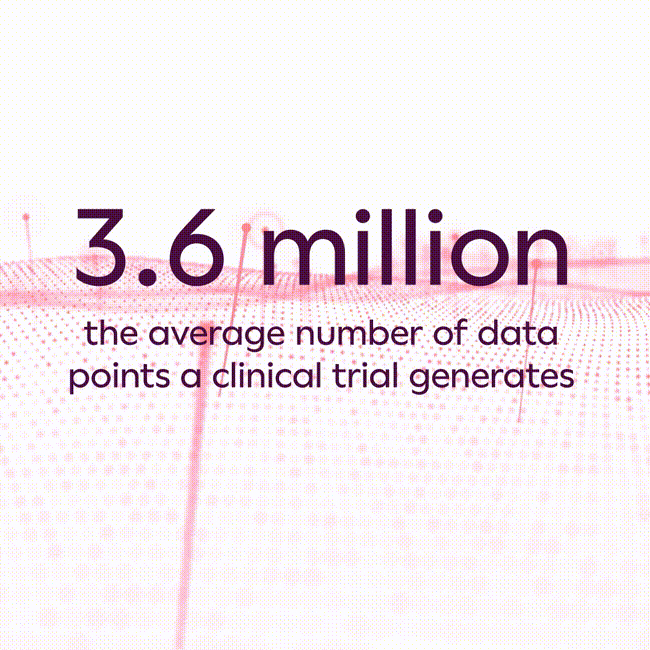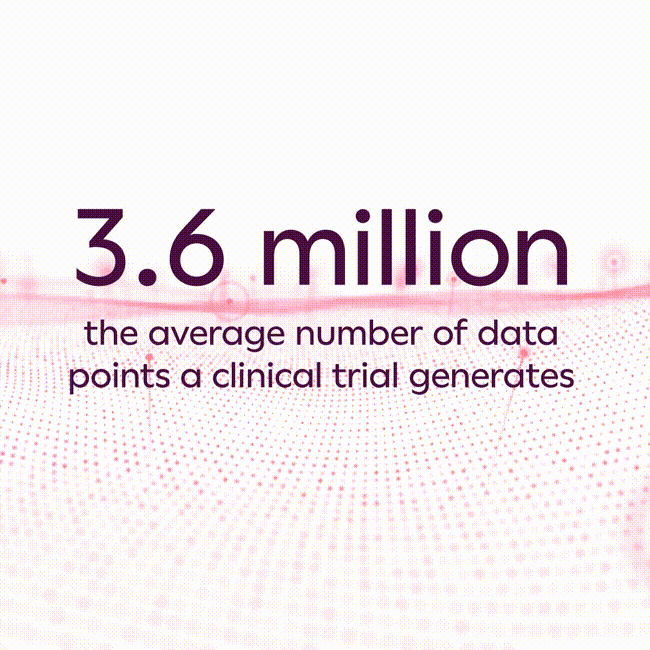
The average clinical trial generates 3.6 million data points1, and that number has increased substantially in recent years due to increasing clinical trial complexity, new endpoint collection technologies, and the rise of hybrid and decentralized clinical trials. As a result, most life sciences companies struggle to scale traditional approaches commonly used in collecting and analyzing data to avoid errors, which can lead to inaccurate study results, unexpected costs, and substantial development program delays.
Many clinical trial technology companies claim to offer AI capabilities, but they often simply run the input data through their own programmed algorithms. With limited quality control or scientific oversight, the responsibility for de-identifying, organizing, and analyzing study data is left primarily with clinical operations and data management teams.
1Tufts Center for the Study of Drug Development
What we talk about when we talk about AI
As the leader in clinical research digital data management, Clario saw the need to transform the way that sites and sponsors were collecting and analyzing these massive amounts of data.
That’s why since 2018 Clario has applied more than 50 AI-enabled solutions across our technology platform that enhance our scientists’ data endpoint analyses by:
- Expediting data collection
- Decreasing variability
- Strengthening patient privacy
- Increasing quality and precision of data
Our AI-enabled data collection and analysis solutions are seamlessly integrated into our platforms and paired with scientific oversight to revolutionize the speed, accuracy, and efficiency of clinical trial endpoint analyses.
Three intelligent tools
One powerful solution
Clario is currently using its state-of-the-art Artificial Intelligence (AI), Machine Learning (ML) and Deep Learning (DL) capabilities across more than 600 ongoing, active clinical trials.
By combining our AI tools with deep scientific expertise, we can achieve faster and more accurate results across a wide range of digital data types while keeping efficiency and scalability at the forefront.
600
Ongoing, active trials using AI
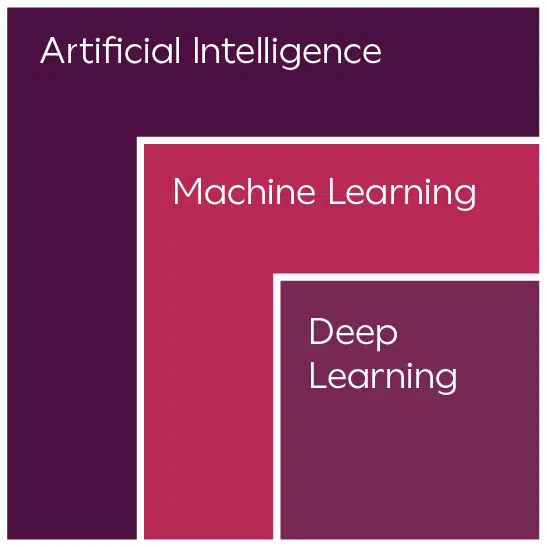
Artificial Intelligence (AI)
Takes the translation learning from Natural Language Processing (NLP) and the data from ML to suggest or take action on the data.
Machine Learning (ML)
This learns from the data. The more it is exposed to, the smarter it becomes.
Deep Learning (DL)
Including computer vision, natural language processing, and speech recognition.
Key benefits
Strengthen patient privacy
21 CFR Part 11 and EU GDPR compliant AI-driven de-identification of PHI and PII with expert human oversight
Increase data quality
Continuous quality assessments and rapid identification of poor-quality data
Faster data analysis
Various AI models quickly collect and support analysis of a wide range of digital data types, leading to shorter study times
SOLUTIONS
The AI solutions across Clario’s technology platform include:

Imaging
Receipt, processing, quality control, and analysis at scale
Powerful AI tools and frameworks are able to perform:
- Complex document and image classifications, segmentations, landmark detections, and highlight regions of interest
- Automatically identify more than 10,000 image series (e.g., MRI, X-ray) a week
- Facial masking enhances patient privacy and makes images available to readers sooner
- Automated motion detection to chart image quality metrics
Cardiac Safety
Increase data quality and reduce the risk of missing data
AI-supported quality assessments for continuous 12-lead ECG recordings, permitting the rapid identification of poor-quality recordings and enabling the opportunity to address any issues directly at the site level quickly enough to implement change.


eCOA
Improve patient safety and data quality
The eCOA Science risk monitoring analytics solution identifies potential issues early during clinical trial conduct, enabling the ability to take timely corrective action. Consistency checks across multiple eCOA instruments are also in place to ensure more robust scientific data and improved investigator oversight and experience.
Respiratory
Decrease end-point variability from poor data quality due to submaximal patient effort
AI algorithms can now automatically analyze spirometry loops to support immediate quality assessment, assessing for maximal patient effort, detecting technical errors, and ensuring consistent best-practice data collection across clinical sites and trial environments. Potentially problematic flow-volume loops can be flagged for further review by a trained specialist and actioned appropriately.

AI responsible use principles
Clario is committed to employing AI in a responsible way governed by the highest ethical principles.
Clario uses AI in a fair and equitable way
To prevent and mitigate the potential of biased or unfair results, Clario uses the most diverse data available to train the algorithms incorporated into our products and services.
Clario is transparent in its use of AI
To ensure transparency and accountability, Clario can explain in plain language: (a) the intended purpose (i.e., objective) of the AI; (b) the data inputs used for training and maintenance of the algorithm(s) used; and (c) what decisions are made by the AI (if applicable) as well as how those decisions are made.
Clario respects privacy rights when using AI
Clario takes seriously its obligations to comply with global data privacy laws and regulations. Clario uses Anonymized Data to train models and algorithms underlying AI. Data used for AI is not traceable back to any individual, clinical trial study, or study sponsor.
Clario monitors and tests AI to detect and mitigate risks
Clario integrates human oversight and monitoring into its development and use of AI solutions to monitor outputs, detect potential bias, and ensure the outputs are fair and reliable.
Clario monitors the evolving AI regulatory landscape for compliance purposes
Clario continuously monitors the rapidly changing regulation of AI and is committed to complying with applicable regulations as they come into force.
Latest AI news
Clario’s New AI-Powered ECG Quality Score Tool Enhances Cardiac Safety Assessments
November 28, 2023A checklist for unlocking the promise of AI in clinical trials
August 22, 2023Transforming clinical trial endpoint analysis: Clario implements over 30 AI solutions to deliver faster and accurate results
May 23, 2023Talk to a specialist
Our team of experts is available to address any questions you may have. Submit your contact information and we’ll be in touch shortly.
