Early precision QT
Minimize risk and make smart decisions
Build confidence with more precise QT assessments
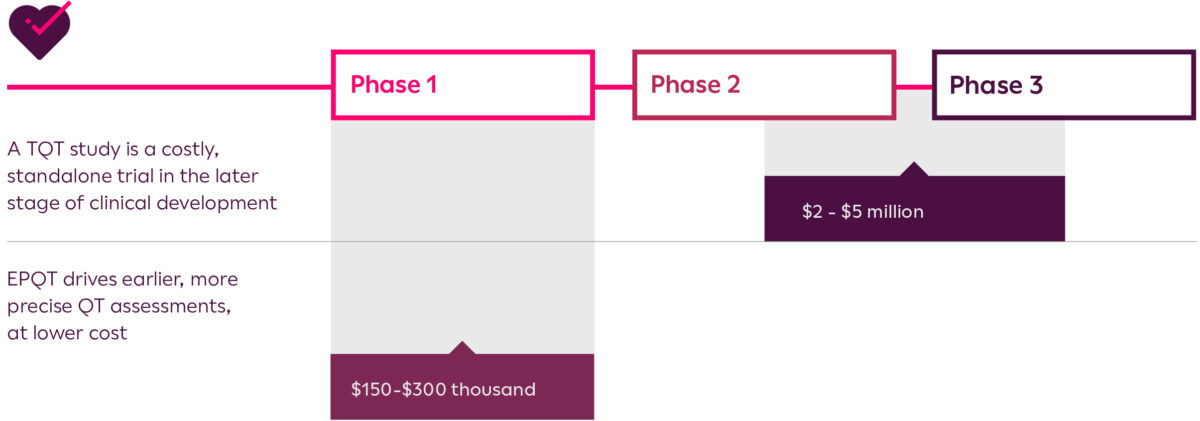
Clinical trials are becoming increasingly complex and study methodologies must evaluate a novel drug in a way that provides a reliable evaluation of its potential benefit. Innovations in cardiac safety assessments could allow for much earlier study of proarrhythmia risk, eliminating the need for costly Thorough QT (TQT) studies in the later stages of clinical trials.
Build confidence in your study data and eliminate risk by driving earlier, more precise QT assessments with Early Precision QT (EPQT). You could save millions in development costs and shorten time to market by getting more granular insights in the initial stage of your development pipeline.
Every millisecond of precision counts when assessing cardiac safety
Obtain highly precise ECG data
Cardiac Technologies, acquired by Clario in late 2017, formulated the transformative EPQT methodology for obtaining highly-precise ECG data, which was developed in collaboration with industry-leading cardiac safety experts, pharmaceutical researchers, the FDA and IQCSRC. The EPQT methodology was clinically proven in a comprehensive clinical trial, which concluded that ECGs collected and analyzed during routine early-phase studies could reliably provide cardiac safety information typically derived from dedicated TQT studies. Confidently implement EPQT in your Phase 1 studies to enable earlier, more precise and cost-effective cardiac safety assessments and potentially qualify for a TQT waiver.
Potentially save millions in TQT study costs while obtaining reliable data earlier in development
Conduct early assessments and comply with regulatory changes
Manage your pipelines more efficiently by enabling earlier product launch and ensuring promising drugs aren’t inappropriately eliminated due to inaccurate cardiac safety data.
Minimize risk early and save resources
In addition to saving precious time and resources, precise QT assessments early in your study can lead to relaxed QT inclusion criteria, which improves patient enrollment. This actionable QT data can be leveraged for out-licensing and improved pipeline prioritization.
Increase confidence with science and expertise
Boost confidence in your trial results by letting our Phase 1 QT Center of Excellence guide you from protocol through final report. Improve data quality and precision with tools that blend the best of technology and medical expertise. We have more Phase 1 QT experience than any other ECG lab in the world — experience gained across 3,600+ Phase 1 cardiac safety trials.
Get precise anticipatory oversight
Ensure the reliability of your early-phase QT assessment with our proprietary Method Bias Sensitivity (MBS) analysis, built and scientifically validated over many trials. With MBS, regulators will have certainty that the ECG core lab has not introduced bias into test results.