Business Intelligence Standard Reports Data Sheet
Reporting and analytics for advancing multicenter imaging
Sponsors and CROs involved in multicenter trials face many challenges related to their studies’ imaging operations. Image acquisition and processing delays along with the time and costs associated with patient image de-identification prolong timelines and burden study sites. Based on 50 years of experience of innovating clinical trials, Clario’s Business Intelligence Suite provides the insights needed in nearly real time, allowing study monitors to recognize potential issues quickly and take corrective action to keep studies on track.
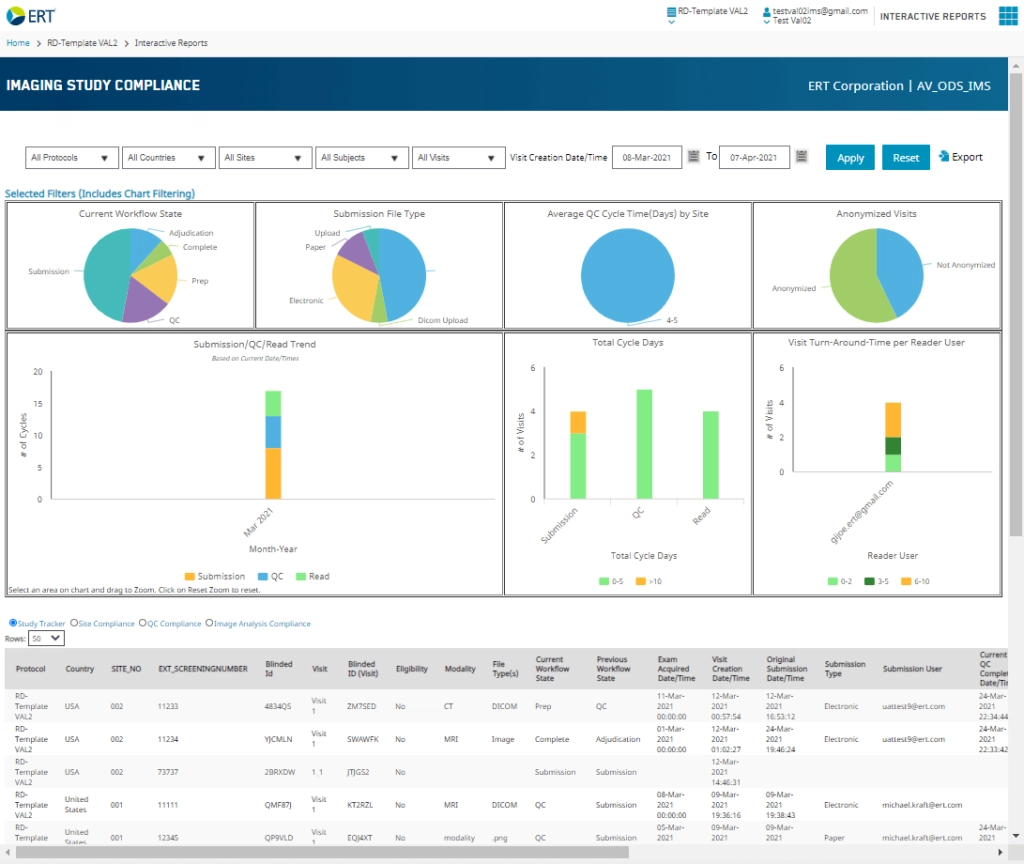
With Clario’s Business Intelligence Suite, comprehensive insights of your imaging study are available at-a-glance. CROs and sponsors can monitor performance and progression of all imaging activities via intuitive, configurable and easy-to-digest dashboards. With this level of easy access to imaging study data, study leaders are empowered to optimize productivity, effectively retrain sites, control costs and ultimately drive study completion.
Unsurpassed accessibility to operational data
Our Business Intelligence Suite offers the perfect combination of detail and ease of use. Dashboards provide high-level views of the process, making regular tracking simple while simultaneously allowing for granular views into site activity, cycle times, throughput and reader performance.
- View workload balance to address potential process backlogs
- Quickly pinpoint missed tasks that are creating redundancies and incremental cost
- Determine if process stages completed in a timely manner
- Assess reader productivity
- Easily identify if workload is at steady state according to study life cycle
- Analyze details provided in table format to investigate abnormal dispersion of cycle times and throughout