Dr. Vince Clinical Research, Clario Form Strategic Partnership to Deliver Innovative Cardiac Assessments in Clinical Trials
- Strategic collaboration amplifies accessibility to Clario’s Early Precision QT (EPQT) methodology, enabling biopharmaceutical firms to secure more accurate and cost-efficient cardiac safety data in early clinical development stages.
- Meticulous multi-stage evaluation positions Dr. Vince Clinical Research as an ideal candidate for intricate trials, leveraging integrated technologies, state-of-the-art telemetry, and deep clinical expertise.
- In many cases, data collected through the partnership can sufficiently fulfill ICH E14 regulatory requirements and qualify Sponsor companies for a Thorough QT study waiver.
OVERLAND PARK, Kan. & PHILADELPHIA, pa.; August 29, 2023 – Dr. Vince Clinical Research (DVCR), an early phase Clinical Research Organization (CRO), today announced a new collaboration with Clario, a leading healthcare research and technology company that delivers leading endpoint technology solutions for clinical trials.
The partnership will extend access to Clario’s clinically proven Early Precision QT (EPQT) methodology. In many cases, this data can sufficiently fulfill International Council for Harmonization (ICH) E14 regulatory requirements and qualify Sponsor companies for a Thorough QT study waiver. Together, Dr. Vince Clinical Research and Clario will empower more biopharmaceutical companies to obtain precise and cost-effective cardiac safety data earlier in the clinical development process.
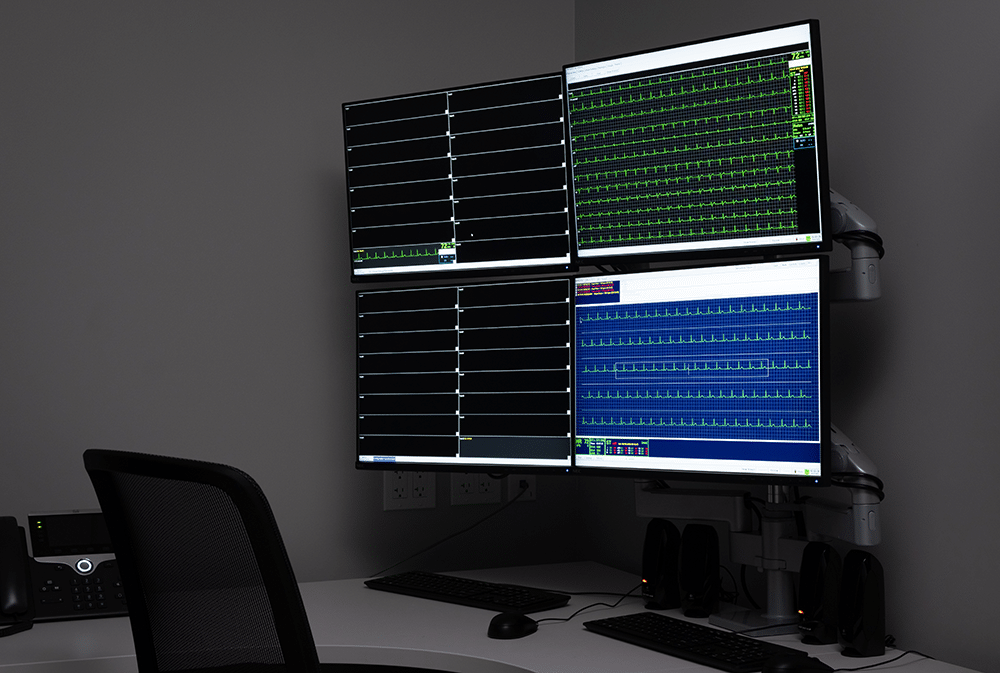
“In order to initiate this strategic partnership, DVCR’s Phase I clinical pharmacology unit underwent a rigorous evaluation process to become a Clario Certified Site,” Robert Kleiman, M.D., Chief Science and Regulatory Advisor, Cardiology, Clario, commented, “It was quickly obvious that the clinical pharmacology unit built by Dr. Vince Clinical Research is exceptionally well- suited to conduct these complex trials given the site’s integrated technologies, state-of-the-art telemetry system, and deep clinical experience. Their organization is already skillfully leveraging Clario’s platforms to provide high-quality cardiac data to our clients.”
Dr. Kleiman added, “The certification process involves collecting continuous cardiac data in a mock clinical trial environment, in accordance with Clario’s guidance documents and using their standardized data collection forms. The collected data was analyzed by Clario’s experts to ensure that each extraction timepoint meets their robust standards for data quality and integrity. As a result, two of DVCR’s personnel were designated as Site Champions.”
The partnership with Clario makes use of the latest technology. The Wi-Fi-enabled 12-lead telemetry system archives data on-site and directly uploads it to Clario’s cloud-based portal. Site staff can remotely monitor tracing quality, reducing data collection interruptions, and improving the experience of study volunteers by reducing the number of electrodes placed on them, leading to improved volunteer retention.
Brad Vince, D.O., DVCR’s CEO & Medical Director, stated, “The successful qualification of our Phase I unit as a Clario Certified Site represents multiple years of planning and a commitment to being a trusted partner in this space. We are excited to be working alongside Dr. Kleiman and Dr. Darpo to leverage Clario’s expertise in cardiac safety.”
The partnership between Clario and DVCR marks a significant milestone in the clinical research industry, setting a new standard for cardiac safety data collection during Phase 1 clinical trials.
About Clario
Clario is a leading healthcare research and technology company that generates high quality clinical evidence for our pharmaceutical, biotech, and medical device partners. We offer comprehensive evidence generation solutions that combine eCOA, cardiac solutions, medical imaging, precision motion, and respiratory endpoints.
Since our founding more than 50 years ago, Clario has delivered deep scientific expertise and broad endpoint technologies to help transform lives around the world. Our endpoint data solutions have supported clinical trials over 26,000 times in more than 100 countries. Our global team of science, technology, and operational experts have supported over 60% of all FDA drug approvals since 2019.
For more information, go to Clario.com or follow us on LinkedIn.
Media Contact:
[email protected]
About Dr. Vince Clinical Research
Dr. Vince Clinical Research (DVCR) is a world-class early phase CRO (Contract Research Organization) with a custom-built, green-designed headquarters and research complex encompassing three buildings in Overland Park, KS. DVCR’s complex includes a 90-bed clinical pharmacology unit featuring a combination of luxurious and private research suites and a cGMP compliant pharmacy. DVCR specializes in conducting clinical trials for both healthy normal volunteers and patient populations in a wide variety of early phase trials. Support services include project management, data management, biostatistics, monitoring and medical writing. By leveraging both technology and one of the country’s most experienced leadership teams, DVCR provides Smarter, Faster Data to their biopharmaceutical clients.
For more information, go to sponsors.drvince.com. Connect with Dr. Vince Clinical Research on LinkedIn and YouTube.
DVCR Contact
Zach Bader
Vice President, Business Development (913) 333-3000
Dr. Vince Clinical Research [email protected]