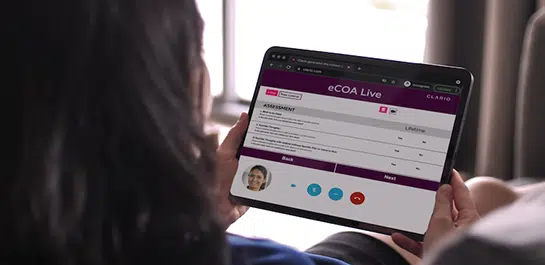
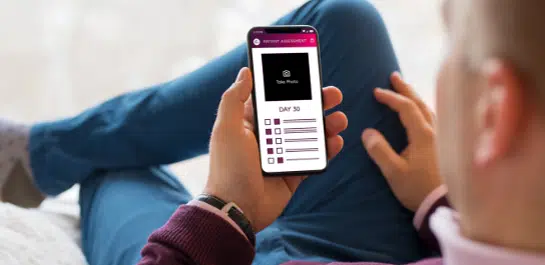
Rapid trial setup
Clario’s eCOA Rapid Start leverages pre-validated assessments to provide a reliable, standardized approach to clinical trials, reducing the burden on clinical teams, and ensuring predictable timelines, all while maintaining the highest quality and compliance standards. As the leading provider of eCOA assessments globally, we’re the partner you can trust to move your study forward, fast.
Clario’s eCOA Rapid Start solution gets you from initial kick-off meeting to user acceptance testing (UAT) in as little as 6 weeks.
Many vendors talk about “libraries” but this simply means reusing previous studies. With Clario’s Rapid Start Catalogue, your study will be using the most up-to-date copyright versions with a reliable version control process, translations, automated testing and use across all modalities and devices.
Rapid start your clinical trial and data capture today
Why Clario eCOA Rapid Start?
Achieve speed, precision, and cost-efficiency with our pre-validated assessments
Fast, predictable timelines
From kick-off to UAT in as little as 6 weeks
Proven, reliable quality
Take advantage of our proven, standardized eCOA assessments to capture the highest-quality data
Cost-effective and ease of set-up
Simplify your study setup and control costs with our pre-validated assessments
Leverage 25+ years of eCOA experience
130+
regulatory approvals
315+
sites worldwide
2.8M+
patients engaged
5.8K+
trials supported
Start using Clario's full range of electronic data capture solutions