Our in-house, cardiac safety consultants optimize clinical trial protocol design, ensure data accuracy and reduce portfolio risks. They have worked with regulatory authorities for decades and have a thorough understanding of what methodologies and processes should be employed to ensure quality outcomes, and as a result, can guide drug development through cardiac safety regulatory requirements.
Our consulting services include:
- Expert protocol development and study design
- Statistical analysis, including concentration QTc analysis
- Authoring expert cardiac safety reports; support for Thorough QT Study waivers
- Regulatory strategy advice and support for health authority interactions
Product detail
Regulatory Consulting
Avoid safety and regulatory issues long before they become critical with strategic protocol and study design advice for early- and late-phase studies. Our consulting team can also help you determine the best devices and integrated solution and select the best strategy for analyzing cardiac safety.
Statistical Analysis Expertise
A team of biostatisticians and statistical programmers with expertise in cardiac safety analysis methodologies facilitate clinical trials through all phases of development.
- Developed tailored cardiac safety analysis plans designed to meet sponsor needs and study design considerations
- Expertise in the analysis of all types of cardiac safety data: QT interval data, ECG morphologic evaluations, continuous Holter / Patch arrhythmia data, 24-hour ambulatory blood pressure data, heart rate variability
- Creation of CDISC compliant analysis datasets for global regulatory submission, including electronic submission deliverable packages
Related solutions
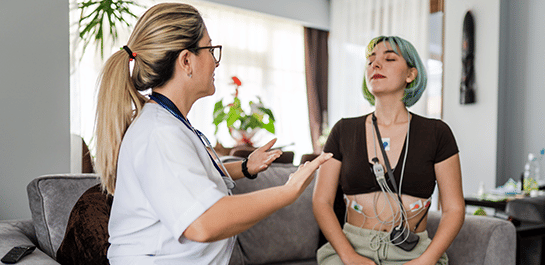
Arrhythmia Analysis
Collect continuous ECG data with certainty in site-based or decentralized clinical trials with our flexible technology solutions and patient-friendly devices.

Blood Pressure Services
Collect and centrally analyze accurate, consistent, quality blood pressure data from site-based to decentralized trials.

ECG — On-site to DCT and Phase I-IV
Monitor every heartbeat with precision from anywhere to assess safety and efficacy throughout your site-based or decentralized clinical trial.
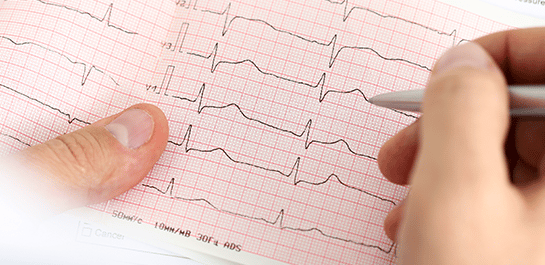
Phase I/TQT Cardiac Assessment
Fulfill your ICH E14 requirement early, minimize risk and potentially reduce costs throughout your trial by collaborating with our Phase 1 Center of Excellence.
Learn more or speak with one our experts
Our team of clinical trial cardiac solution experts is always available to address any questions you may have about our cardiac solutions. Submit your contact information and we’ll be in touch shortly.
