Situation
A leading global pharmaceutical company initiated a clinical trial for a new asthma compound, leveraging standardized, centralized spirometry. During the study, there were large discrepancies in spirometry results produced by investigative sites from subjects’ baseline inclusion measurements when compared to later measurements under treatment.

Impact
Weekly custom reports with alerts identified intra- and inter-visit variability in FEV1 % and raw mL changes. Throughout the study, 37% of active sites received a C or lower grade in data quality, prompting further investigation. 24% of patients screened into the study were identified with out-of-range spirometry tests at over 50 different sites. Additionally, 3% of study subjects were deemed to demonstrate inexplicably high reversibility data. Armed with these insights, the study clinical team was able to effectively follow up with all responsible investigators.
Some sites were put on recruitment hold and closely monitored until rapid improvement was made. Enhanced Business Intelligence recognized sites that used different patients to perform baseline and treatment spirometry, sites that produced questionable data that still met ATS/ ERS standards (and were thus difficult to detect) and sites that were not in compliance with protocol requirements. The most critical sites with sub-optimal performance were retrained remotely or onsite to drive improvement in their decision-making process and overall data quality.
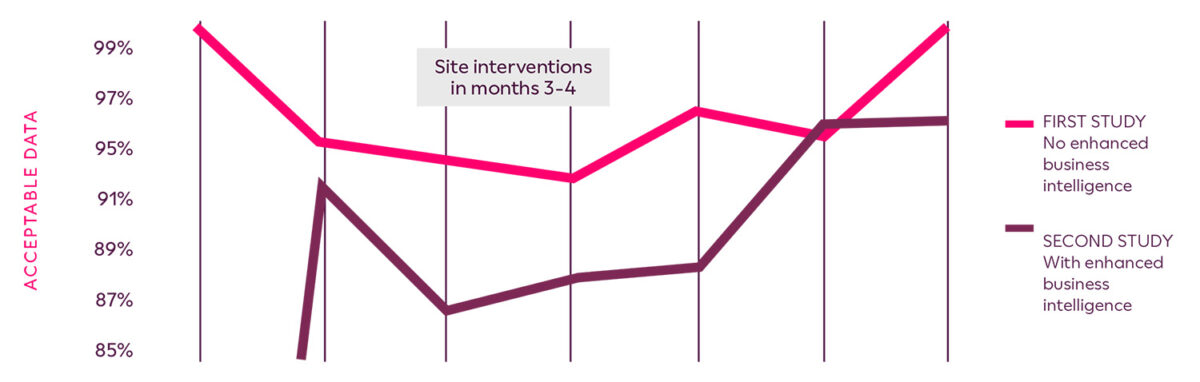
Ultimately, acceptable data between the first study and the second study was increased by 4%. The ability to quickly recognize suspect or problematic data provided an additional level of assurance in regards to the data integrity and reliability of the primary endpoint. The sponsor achieved database lock on schedule and generated higher quality data for regulatory submission.
Enhanced Business Intelligence for Respiratory Trials custom reports identifies low-performing sites and drives site intervention activities to stabilize data quality above 95%.
Clario enabled a 4% increase in data acceptability, above 95% overall