- Home Resources Blog Articles Total metabolic tumor volume — A valuable new method of assessment of global response to therapy
Total metabolic tumor volume — A valuable new method of assessment of global response to therapy
This measurement brings useful, independent, and correlative information to the clinical drug evaluation process
John G Wolodzko, Ph.D., DABSNM, FACNM – Sr. Medical Physicist at Clario
Over the past few years, many papers have been published by groups investigating the utility of 18F-fluorodeoxyglucose-Positron Emission Tomography (FDG-PET) for screening and therapy response assessment in various lymphoma subtypes.
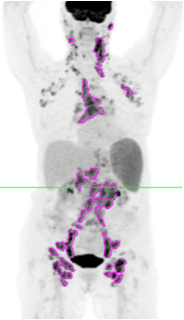
Several of these studies have reported the value of total metabolic tumor volume (TMTV) as a negative or positive prognostic indicator, based on a TMTV above or below a predetermined threshold at baseline, or as an indicator of response according to a change in TMTV over a course of treatment. Different approaches have been investigated for segmenting lesions for volume measurement, including the setting of image thresholds at fixed percentages of lesion maximum standard uptake values (SUVmax), e.g., the 41% of SUVmax suggested by the European Association of Nuclear Medicine (EANM); absolute SUV, e.g. SUV>= 4.0 as advised by the PETRA group; or a multiple of a reference value such as 2.5 times the Liver SUVmean. Although more than one group has concluded that the selection of the most sensitive baseline TMTV cutoff for the purpose of prognostic determination appears to be disease-specific, there is consensus that most, if not all, of the approaches to threshold setting have similar prognostic or response results when the selected method is applied consistently. In addition, at least one group of researchers have reported that FDG-PET-based TMTV evaluations are superior to approaches such as measurements of anatomical size or bulk or qualitative PET assessments such as numerical scoring systems in terms of predicting treatment outcomes or demonstrating response to treatment. TMTV measurements can potentially aid in the selection of patients for appropriate therapies or may support the modification of drug doses for maximum effectiveness.
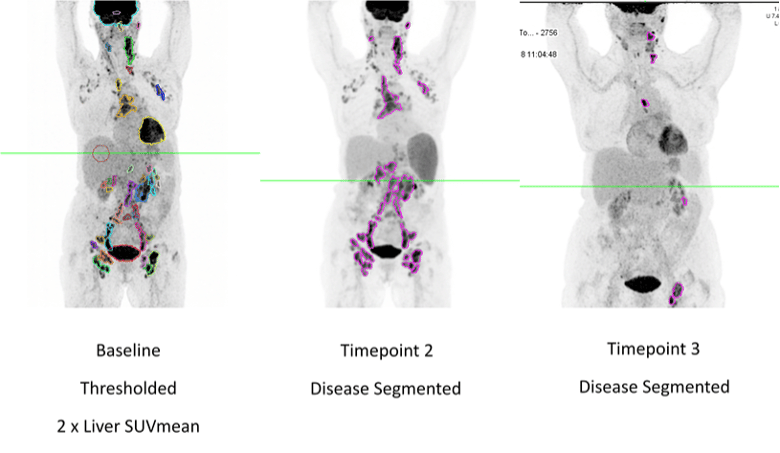
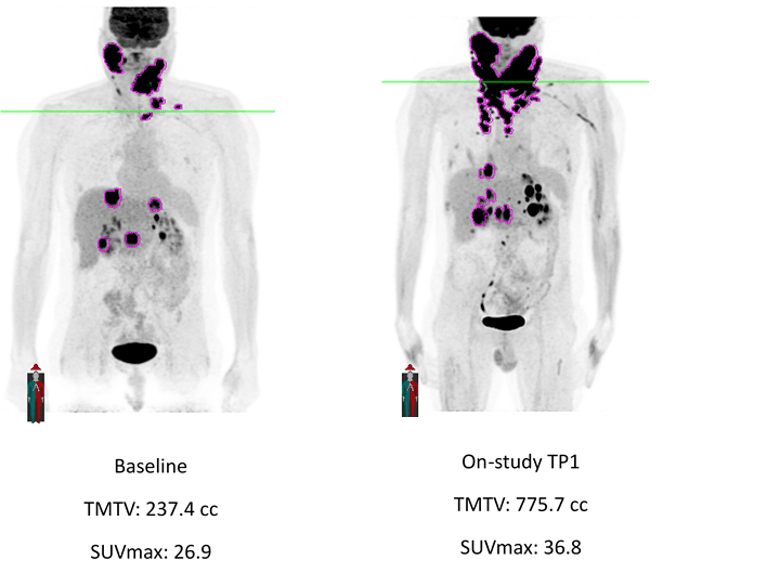
FDG-PET-based TMTV measurement brings useful, independent, and correlative information to the clinical drug evaluation process. Clario can customize the segmentation threshold settings to meet any special requirements based on disease type or sponsor request or provide measurements based on multiple approaches to segmentation for sponsor downstream evaluation if desired. Additional data, such as Total Lesion Glycolysis (TLG), Fragmentation, and Dissemination based on metabolic tumor volume and distribution of lesions can also be provided for prognostic/response evaluation and can be included in the data provided to the sponsor for a comprehensive demonstration of drug efficacy.
Explore further
Deepen your understanding of oncology and the groundbreaking advancements in the field. Let Clario guide you through the latest research, findings, and innovations.
Written by

John G Wolodzko, Ph.D., DABSNM, FACNM
Sr. Medical Physicist at Clario
References
1Cottereau A.-S., Hapdey S., Chartier L., Modzelewski R., Casasnovas O., Itti E., Tilly H., Vera P., Meignan M., Becker S. Baseline Total Metabolic Tumor Volume Measured with Fixed or Different Adaptive Thresholding Methods Equally Predicts Outcome in Peripheral T Cell Lymphoma. J. Nucl. Med. 2016;58:276–281
2A. S. Cottereau, S. Becker, F. Broussais et al., “Prognostic value of baseline total metabolic tumor volume (TMTV0) measured on FDG-PET/CT in patients with peripheral T-cell lymphoma (PTCL),” Annals of Oncology, vol. 27, no. 4, pp. 719–724, 2016
3Burggraaff CN, Rahman F, Kaßner I, Pieplenbosch S, Barrington SF, Jauw YWS, et al. Optimizing workflows for fast and reliable metabolic tumor volume measurements in diffuse large B cell lymphoma. Mol Imaging Biol. 2020;22:1102–10