Clario Announces Real-Time Coaching for Pulmonary Function Tests Performed in the Patient’s Home
Groundbreaking technology enables virtual spirometry visits for decentralized or hybrid clinical trials
PHILADELPHIA – June 8, 2021 – ERT, the global leader in data collection for clinical endpoints, today announced the launch of ERT iSpiro Virtual Visits, which enables real-time coaching during at-home pulmonary function tests (PFT). With iSpiro Virtual Visits, ERT delivers on its commitment to provide customer and patient-oriented innovations that facilitate decentralization.
The clinical trials ecosystem is evolving rapidly, and the need for reliable remote data collection is increasing. Correct performance of a spirometry measurement without the guidance of a specially trained nurse is a challenge, primarily when documenting minor differences measured in a few milliliters of volume. iSpiro Virtual Visits keeps respiratory clinical trials on track by enabling high-quality spirometry data collection at home, even when a patient cannot visit a clinic.
“Performing spirometry correctly can be critical to the success of a drug approval,” said Achim Schülke, Executive Vice President of ERT Respiratory Solutions. “With iSpiro Virtual Visits, it is now possible to test patients from home without compromising on data quality or patient safety.”
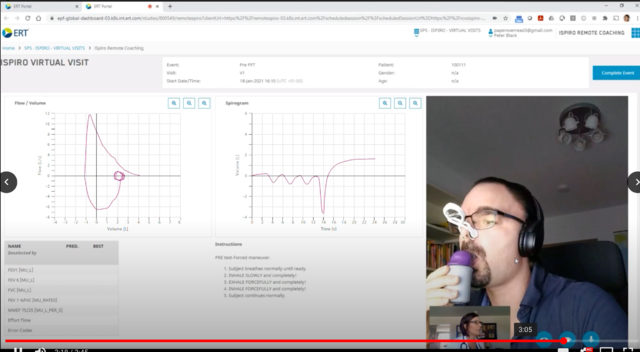
iSpiro Virtual Visits is the only existing solution that, in compliance with regulatory requirements and in-clinical quality, enables guided lung function assessment at home via the patient’s smartphone. The solution incorporates the iSpiro smartphone app from ERT with a highly accurate, user-friendly spirometry sensor approved for home use. While the nurse and patient go through the effort-dependent spirometry measurement step-by-step via live video, a real-time spirometry signal is simultaneously shared on the screen, thus enabling the nurse to execute optimal coaching.
To find out more about iSpiro Virtual Visits and to receive a demo, contact us at [email protected].