Clario Enhances Business Intelligence Suite with Clinical Imaging Reporting Capabilities
Unmatched data visibility accelerates the informed decision-making process for CROs and study sponsors
PHILADELPHIA — April 14, 2021 — ERT, the global leader in clinical endpoint data collection, today announced that its proven Business Intelligence suite has expanded to include reporting for clinical trial imaging, making study data more easily accessible and actionable. The new imaging capabilities provide a heightened level of information that enables study leaders to ensure completion, and effectively manage sites, costs, and productivity.
“Imaging studies are complex, and customers are under great pressure to monitor and manage their performance. With the power of our Business Intelligence suite, study leaders can achieve the deep insights they need to act quickly, and keep studies on track for success,” said Tim Kulbago, Vice President, Imaging, ERT. “Our clinical trial imaging customers can now track their study progress all the way from first image capture to database lock, with the ability to pivot with confidence whenever a potential challenge arises.”
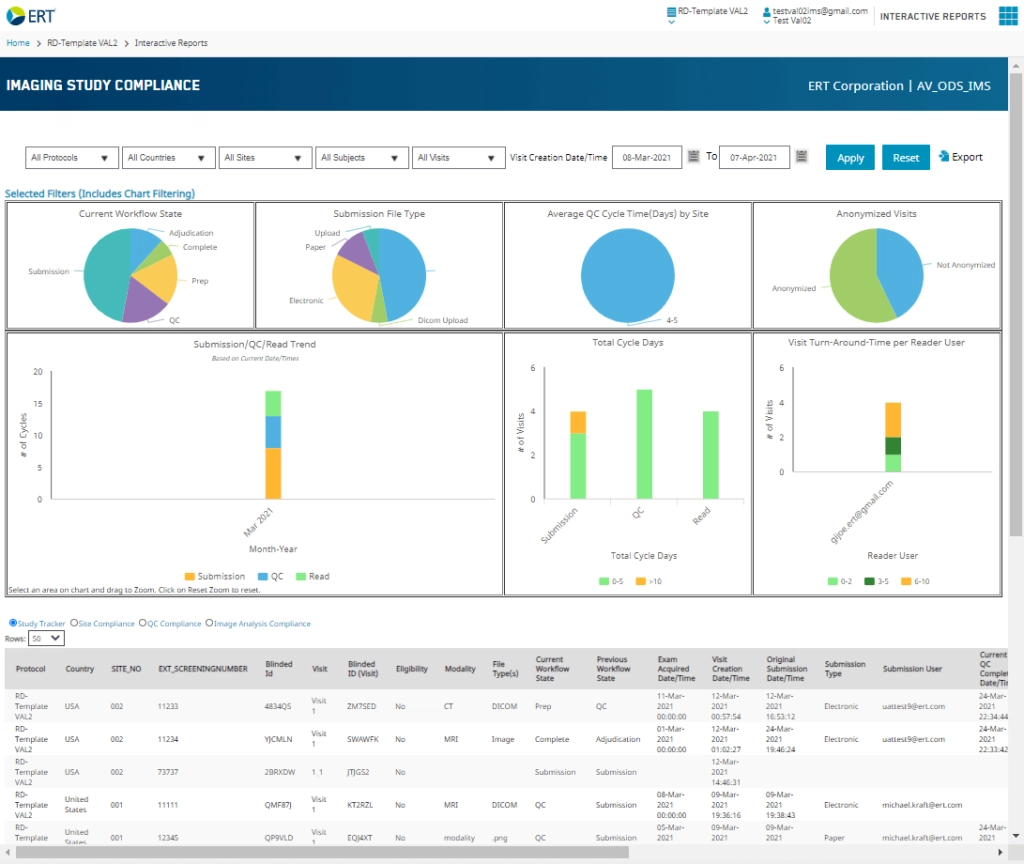
ERT’s Business Intelligence suite allows many CROs and sponsors to realize new operational efficiencies and better data quality, along with reduced study timelines and lower costs, through its easily digestible and comprehensive views into trial performance. Customers can use the new imaging functionality to direct studies using a depth of information never before available. With the new capabilities, they have access to an easy-to-understand dashboard for assessing early indicators of potential delays in the imaging process.
Users will find an intuitive interface that allows them to access study data in a variety of ways. They can easily filter data for a high-level view or dive down into specifics by region, site and image reader, among others. Monitoring compliance is simple, as users can quickly see an overall study level or look at compliance in specific areas such as quality control, by site, and even by image reader. Timely information allows study leaders to intervene as necessary, for example, if a site has a high number of queries or if a reader appears to be deviating from the protocol.
About Clario
Clario delivers the leading endpoint technology solutions for clinical trials. Through experience gained from over 19,000 clinical trials delivered in support of 870 regulatory approvals, Clario fuses scientific expertise and global scale into the broadest endpoint technology platform to enable pharmaceutical, biotech and medical device partners to transform lives. Clario has mastered the ability to generate rich evidence across all trial models: decentralized, hybrid and site-based clinical trials. With 30 facilities in nine countries across North America, Europe and Asia Pacific, Clario’s global team of science, technology and operational experts has been delivering the richest clinical evidence for nearly 50 years.
For more information, go to Clario.com or follow us on LinkedIn and Twitter.