Ophthalmology clinical trials
Clario’s ophthalmology therapeutic area supports clinical trials evaluating the efficacy of therapeutics designed to treat the eye as well as other trial monitoring ophthalmic safety events. Similar to our other therapeutic areas, these services span the entire trial lifecycle of your study, from training and qualification, clinical project and data management, and image QC and analysis to regulatory submission.

The Clario/Cleveland Clinic’s Cole Eye Institute partnership
Our collaboration with Drs. Peter K Kaiser, Justis Ehlers, and Sunil Srivistava and their team at The Cole Eye Institute’s Eye Care Center brings the world-class expertise and experience required to provide high-quality endpoint data, enabling you to bring emerging ophthalmic therapies to the market.
Cole has a team of industry-leading ophthalmologists and expert graders that can provide high-quality, fast and reliable ophthalmic image grading. Additionally, the long standing (10+ years) collaboration with Clario includes a generation of AI algorithms that can pre-process and segment images within the clinical trial workflow for a given trial; supplementing the expert graders’ assessments and supporting the generation of new and novel endpoints. Clario brings decades of clinical trial experience and advanced technology with a global footprint and 24/7 support.
Expert support throughout the trial lifecycle
When you partner with Clario for your ophthalmology trial, you’ll receive a dedicated, comprehensive approach to all aspects of your study. We pair deep clinical expertise with our broad experience in protocol design, site management and patient support capabilities to provide you with powerful solutions to optimize your study.
- Protocol review: Ensure imaging modalities and associated parameters are appropriate and feasible for the proposed study endpoints and provide recommendations on additional exploratory endpoints that may supplement post-approval marketing messaging.
- Study design: Design studies to meet your needs both in terms of operational efficiency and regulatory compliance while minimizing patient burden.
- Site and equipment/technologist qualification: Ensure the quality of images generated by equipment and technologists is adequate for assessment of study endpoints with thorough site surveys and a qualification process that includes submission and QC of test imaging prior to the site’s first subject visit.
- Site training: Educate site staff on the acquisition parameters and techniques required for a given modality using online training resources and manuals.
- Clinical Project Management: Project Management teams excel in delivering results tailored to your study, objectives, timelines, metrics and budgets.
- Clinical Data Management: Provide robust data formats and efficient transfer methods that meet industry standards for studies of all complexities.
- Quality Review: Check accuracy and completeness of data (image quality, demographics, anatomical coverage and parameter adherence) at each timepoint to ensure images are adequate for assessment.
- Image Analysis: Generate highly accurate measurements and turn around assessments quickly if required for eligibility/safety using a team of specialized graders.
- Regulatory submission: Assistance in preparing high-quality data, including exploratory findings, for regulatory submission and presentation.
Our Ophthalmology experience and expertise
20
years’ experience
70+
global phase 1-4 trials
4
key opinion leaders
20+
expert graders
Overview
Clario’s new therapeutic area combines 20 years of ophthalmology imaging expertise, with its decades of cross-therapeutic area study experience.
The ophthalmology therapeutic area supports a range of ophthalmic imaging modalities across all major ophthalmology indications, performing qualitative and quantitative assessments, including advanced, fully customizable proprietary quantitative analysis incorporated within a completely configurable, study-specific workflow. Modalities include OCT/OCTA, color fundus (CF) photography, fluorescein angiography (FA) and fundus autofluorescence (FAF) across all major ophthalmology indications.
Clario can tailor its ophthalmic software platform to meet study protocol requirements, including, but not limited to, complex workflow design (parallel or interdependent timepoint/modality workflows), comprehensive QC assessments (supplemented by AI for near real-time quality feedback) and AI-assisted quantitative analysis with customizable per modality grader paradigms (single, 2+1, etc.).
Capabilities
Clario’s technology accelerates the ophthalmology clinical trial process:
- Real-time access to images: View images/download images on-demand with or without grader annotations (customizable permission per role, i.e., site, sponsor and medical monitors) at no additional charge.
- Intuitive site interface for image upload: Upload images quickly via intuitive drag-and-drop functionality.
- Configurable viewers for reader assessments: Vendor agnostic viewer with configurable viewports and annotation/measurement tools to adjust AI-generated pre-segmented objects (layer lines and fluid, etc.).
- Rapid image quality assessment: Near real-time automated quality assessment using AI to notify sites if a rescan is required.
- Centralized imaging platform with multi-modal capabilities: Display images from all modalities and for expedited grading and real-time sponsor access. Aggregation of assessments from multiple modalities (e.g., OCT and Fundus) to determine patient eligibility (automated site notifications).
- Fully customizable multi-modality workflows and reader paradigms: Incorporate 1/2/3 graders and an adjudicator if required, customizable on both a timepoint and modality level.
- Advanced proprietary quantitative analysis: Incorporate proprietary quantitative analysis (AI algorithms) into grader assessment workflows to provide consistency and improve efficiency.
- 21 CFR Part 11 and GDPR compliance: Fully compliant cloud-based platform with verified data integrity checks, access control management, electronic signoffs and a transparent and comprehensive audit trail.
- 24/7 global support and deployment: Keep trials on track with 24/7 global support.
Advanced Quantitative Analysis
Advanced quantitative analysis is now an essential part of ophthalmology trials.
Clario/Cole Eye Institute R&D
Our R&D arm continually develops quantitative analysis algorithms that enable you to perform advanced assessments and generate quantitative novel endpoints for emerging therapies.
AI technology
Our centralized platform incorporates sophisticated AI technology, which generates segmentations for complex, high-volume assessments that would not be practical or cost-effective for graders to accomplish. These include:
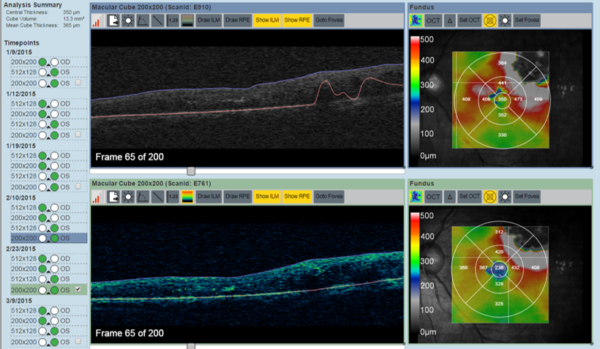
- ILM and RPE layer segmentation for retinal thickness analysis
- Detection of EZ/RPE layers for enface ellipsoidal zone disruption mapping
- Retinal thickness map analysis for multiple layers (ILM, RPE, Bruchs membrane, ONL, etc.)
- Intraretinal/subretinal fluid and subretinal material segmentation for longitudinal volume change
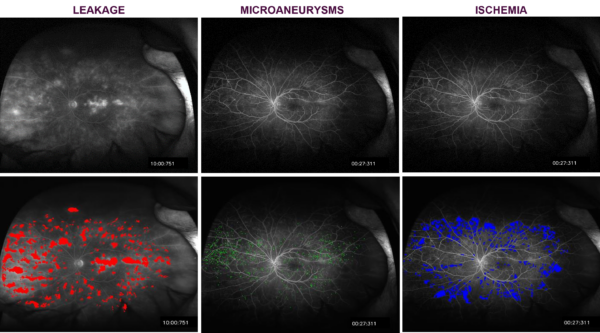
- Evaluation for leakage (perivascular and general), microaneurysm, ischemia and vessel segmentation in fluorescein angiography images
- OCT-based GA and c/iRORA analysis
Our AI technology also rapidly assesses OCT/FA image quality and immediately notifies sites if a rescan is required.
Focus areas
- OCT/OCTA (anterior/posterior, all vendors)
- Color fundus photography (CF) (30-60 degrees and wide-field)
- Fluorescein angiography (FA) (30-60 degrees and wide-field)
- Indocyanine green angiography (ICGA)
- Fundus auto-fluorescence (FAF) (30-60 degrees and wide-field)
- Infrared reflectance
- Slit-lamp photography
- Video analysis (e.g., maze/movement studies)
- Diabetic retinopathy
- Wet/dry AMD
- Uveitis
- Retinal vein occlusion
- Vitreomacular traction
- Epiretinal membrane
- Macular hole
- Inherited retinal degenerations (e.g., retinitis pigmentosa)
- Glaucoma
- Optic neuropathies
- Neurology (MS studies)
- Systemic therapies with ocular side effects
- OCT layer line segmentation
- RPE integrity/geographic atrophy (OCT/FA/FAF/CF)
- CNV location, area, classification (OCT/OCTA/FA)
- Macular edema, subretinal/intraretinal fluid, pigment epithelial detachment, drusen analysis, ellipsoidal zone disruption (OCT)
- Ischemia, leakage, microaneurysms (FA)
- Zonal retinal nerve fiber layer thickness (OCT)
- Cup-to-disc ratio (CDR) (CF)
- Macular hole volume/dimensions (OCT)
- Diabetic Retinopathy Severity Score (DRSS)
- Ischemia, leakage, microaneurysms (FA)
- Zonal retinal nerve fiber layer thickness (OCT)
- Cup-to-disc ratio (CDR) (CF)
- Macular hole volume/dimensions (OCT)
- Diabetic Retinopathy Severity Sc
- OCT/OCTA (anterior/posterior, all vendors)
- Color fundus photography (CF) (30-60 degrees and wide-field)
- Fluorescein angiography (FA) (30-60 degrees and wide-field)
- Indocyanine green angiography (ICGA)
- Fundus auto-fluorescence (FAF) (30-60 degrees and wide-field)
- Infrared reflectance
- Slit-lamp photography
- Video analysis (e.g., maze/movement studies)
- Diabetic retinopathy
- Wet/dry AMD
- Uveitis
- Retinal vein occlusion
- Vitreomacular traction
- Epiretinal membrane
- Macular hole
- Inherited retinal degenerations (e.g., retinitis pigmentosa)
- Glaucoma
- Optic neuropathies
- Neurology (MS studies)
- Systemic therapies with ocular side effects
- OCT layer line segmentation
- RPE integrity/geographic atrophy (OCT/FA/FAF/CF)
- CNV location, area, classification (OCT/OCTA/FA)
- Macular edema, subretinal/intraretinal fluid, pigment epithelial detachment, drusen analysis, ellipsoidal zone disruption (OCT)
- Ischemia, leakage, microaneurysms (FA)
- Zonal retinal nerve fiber layer thickness (OCT)
- Cup-to-disc ratio (CDR) (CF)
- Macular hole volume/dimensions (OCT)
- Diabetic Retinopathy Severity Score (DRSS)
- Ischemia, leakage, microaneurysms (FA)
- Zonal retinal nerve fiber layer thickness (OCT)
- Cup-to-disc ratio (CDR) (CF)
- Macular hole volume/dimensions (OCT)
- Diabetic Retinopathy Severity Sc
Key benefits to sponsors
Bring emerging therapies to market
Enhance regulatory submissions
Improve clinical trial experience
Talk to a specialist
Our team of experts are available to address any questions you may have about our ophthalmology solutions. Submit your contact information and we’ll be in touch shortly.